For the vast majority of life-saving drugs, therapies, and vaccines, efficacy is inextricably tied to temperature stability.
The most common acceptable temperature range for cold chain pharmaceuticals is between 2°C and 8°C. Ensuring temperature stability across thousands of shipments as they move throughout dozens of distribution points requires precise, reliable testing.
The following is a look into how that testing is performed.
Thermal Shipping Regulation: Who’s Who
Effective transport of temperature sensitive products through the small parcel distribution network depends on guidance provided by multiple industry organizations.
The primary organization that provides guidance on the proper qualification of Insulated Shipping Containers (ISC) used for the distribution of temperature sensitive products is the International Safe Transit Association (ISTA).
The International Safe Transit Association, is an industry organization that provides standardized testing methods to members and provides lab certifications for both physical and thermal testing of packaged products.
The American Society for Testing Materials, is another organization with test standards that are used in the lSTA. While we focus more on ITSA in this article, ASTM covers a wide range of test standards and its test methods can be used for shipments destined for either domestic or international transit.
The Food and Drug Administration is the governing body that sets regulations for the proper certification of ISCs for drug manufacturers and distributors.
The Utilization Review Accreditation Commission, described as a “nonprofit accreditation entity”, oversees the validation process and requirements of ISCs used by specialty pharmacies in its 4.0 recommendations.
ISC
ISC is a packaging system as shipped. It typically includes an outer box, thermal insulation, coolant of some kind, and a temperature sensitive payload.

The Guidebook for Thermal Testing:ISTA Standard 20
Due to a lack of standardization in the verification process of ISCs, ISTA established the ISTA Standard 20 (STD-0020), a testing process that outlines the process of design, testing, and verification of an ISC.
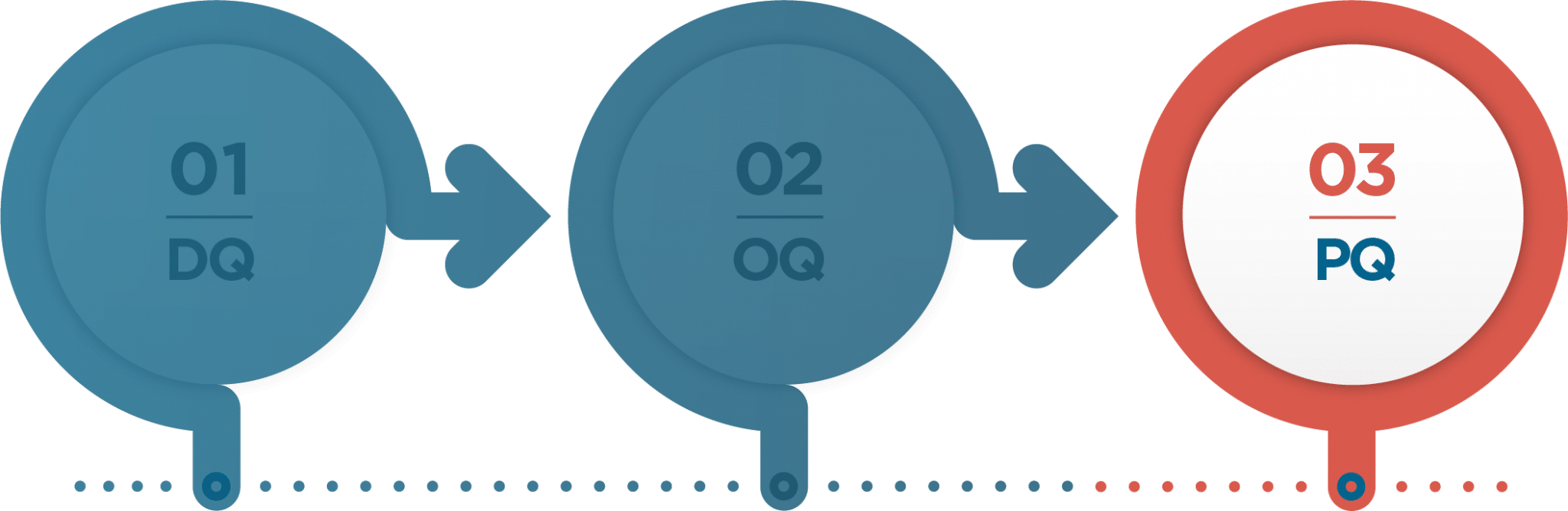
Proper execution of the Standard 20 process will certify that an ISC performs to the specified user requirements in a manner that meets FDA regulations. The Standard 20 process relies on three stages of testing to fully qualify an ISC.

STAGE 1: DQ
The initial stage is the Design Qualification (DQ), which is done either through environmental chamber testing or thermal simulation and only requires a sample size of one.
STAGE 2: OQ
After an ISC passes DQ, the Operational Qualification (OQ) is completed. OQ testing requires a minimum sample size of three and requires thermal chamber data as well as distribution testing.
STAGE 3: PQ
Following the completion of the OQ testing, the ISC is subjected to a Performance Qualification (PQ). The PQ is performed through real world shipments and requires a sample size of three shipments in the most extreme temperatures the intended season.
Standard 20 outlines the proper data collection process for each qualification step, as well as what to include in the final documentation for a fully qualified ISC.
Recreating Real Life in a Thermal Chamber: ISTA 7E
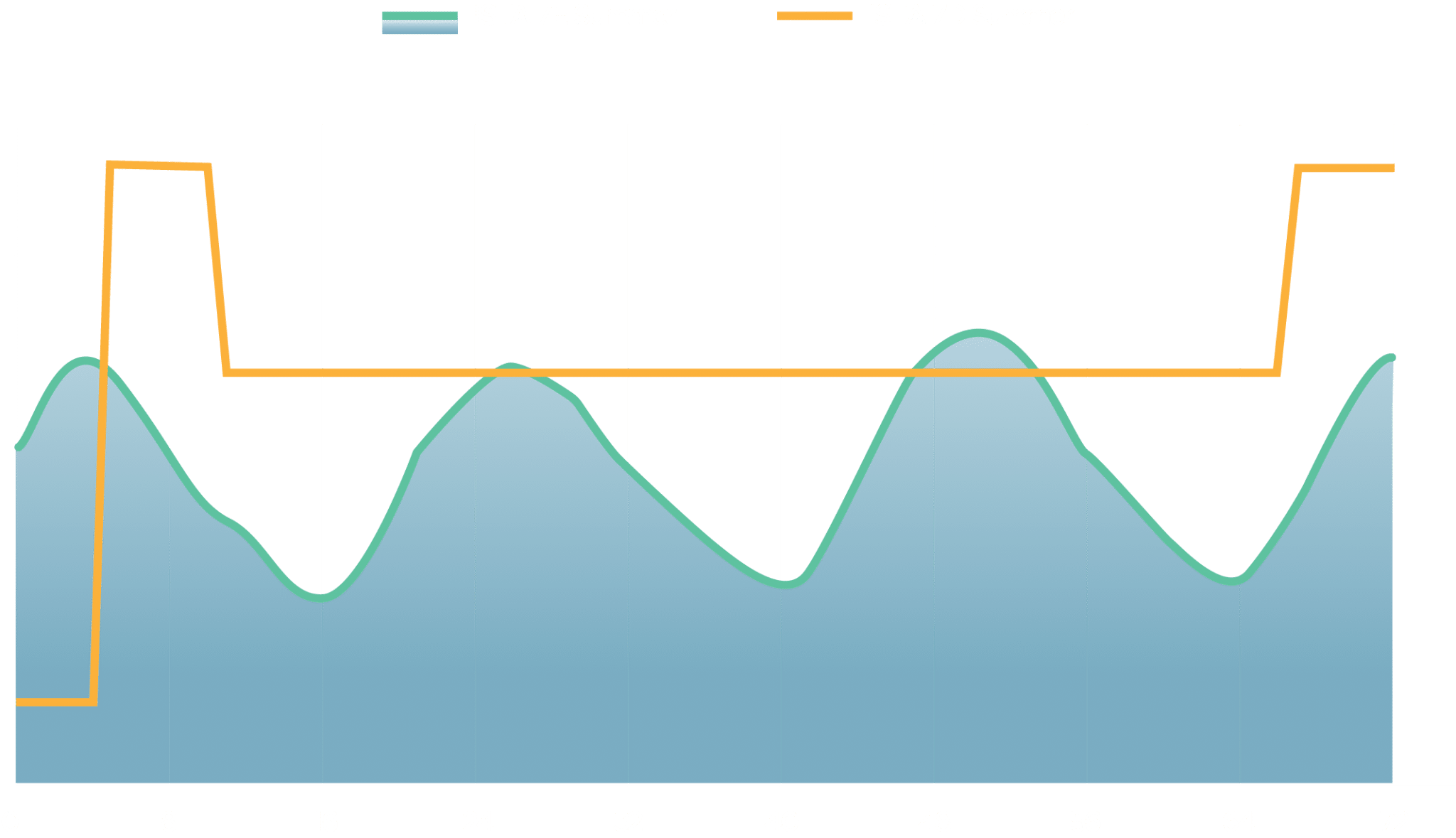
Along with extensive guidance and requirements for the testing process, data collection, and documentation, Standard 20 also references the ISTA 7E thermal profiles for use in chamber testing. The ISTA 7E thermal profiles were developed through industry input to provide standardized methodology for the comparison of ISCs.
They include a standardized summer and winter profile that were built using an aggregation of data collected through an extensive study of shipping lanes spanning the United States. Each 7E profile contains up to 72 hours of data, which is repeatable for up to a total duration of 144 hours.
7E vs 7D
While the seasonal 7E profiles can be used to demonstrate performance for general domestic shipments, it may not reach the temperature extremes observed across specific regions. This is the reasoning for the PQ step in the Standard 20 process, but for many users a custom profile built using specific lane data may be more representative during chamber testing. Some end users address this discrepancy in temperature extremes by requiring ISTA 7D as the baseline for initial testing, with the intention of validating later down the road using real temperature data collected across specific temperature lanes to perform an OQ and following it up with a PQ.
A simple examination of 7E vs 7D will show the contrast between the two profiles. ISTA 7D was developed prior to 7E. It contains immediate temperature changes in the profile followed by hold/soak steps at a constant temperature, similar to what would be experienced when going from inside a warehouse to outside and vice versa. ISTA 7E was developed using an intensive data collection process, and generally represents the gradual temperature changes that can be experienced throughout the day.
Shake it Won’t Break It: ISTA 3A
The ISTA Standard 20 process also requires the ISTA 3A test, which is described as a “general simulation test for individual packaged-products shipped through a parcel delivery system”. The ISTA 3A involves a series of drops, dynamic compression sequences, and a loose load vibration portion that are meant to replicate the different forces that can be experienced by an ISC or other single shipment when sent through a service like UPS or FedEx.
Specialty Pharmacy:
When drug manufacturers and distributors make a submission to the FDA, both OQ and PQ documentation for an ISC are required to receive approval. However, specialty pharmacies that are subjected to URAC 4.0 requirements are only required to submit PQ data.
While not required by URAC, DQ and OQ testing is recommended to reduce the risk of failure during the PQ phase of the package development process.
Here to Help
When food or medicine requires temperature control distribution, the design and qualification of insulated shipping containers is an essential of new product development.
Through the ISTA Certified Thermal Lab at TemperPack, our team of engineers is able to carry out a standard or modified version of the ISTA Standard 20 process.








